Recently, the team of researchers Haohao Dong of the State Key Laboratory of Biotherapy of our university published a report entitled "Cryo-EM structures of lipopolysaccharide transporter LptB2FGC in lipopolysaccharide or AMP-PNP-bound states reveal its transport mechanism " in the international journal Nature Communications (impact factor 11.878). The main work of the research was completed by the teams of researcher Haohao Dong and Professor Xing Zhang, director of the cryo-EM center of Zhejiang University School of Medicine. The first author of the paper is Dr. Xiaodi Tang, associate professor of the State Key Laboratory of Biotherapy of our university, Dr. Shenghai Chang, engineer of the cryo-EM center of Zhejiang University, Qinghua Luo, a doctoral student of our university, and Zhengyu Zhang, a researcher of the School of Pharmacy of Wuhan University, as the joint first authors. The State Key Laboratory of Biotherapy/National Clinical Research Center for Geriatric Diseases is the first author and corresponding author of the paper.
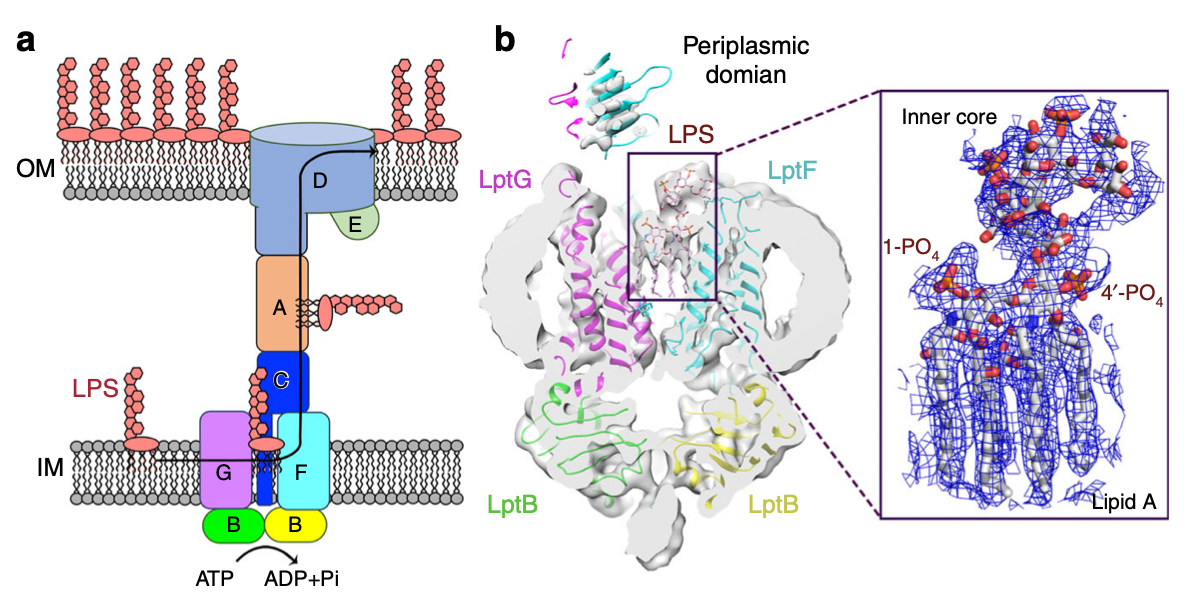
This study reveals the high-resolution cryo-EM structure of the protein machine complex LptB2FGC used by multidrug-resistant bacteria-gram-negative bacteria to transport outer membrane lipopolysaccharides, bound to its substrate lipopolysaccharide or AMP-PNP. Through the analysis of structure and the study of the function in the cell, the important functional sites of the protein machine and its high-efficiency transport mechanism are revealed.
Infections with multidrug-resistant bacteria, or "superbugs," pose a significant threat to human health. The World Health Organization (WHO) has divided the level of urgency for new antibiotics into three categories: moderately important, very important and extremely important, of which the development of new antibiotics against three gram-negative bacteria, Pseudomonas aeruginosa, Enterobacter and Acinetobacter baumannii, is listed as extremely important. The unique outer membrane structure-lipopolysaccharide protective layer of gram-negative bacteria is an important reason for their drug resistance, so destroying the synthesis and transport of outer membrane lipopolysaccharides is an important way to study new antibiotics. The target protein machine of this project, LptB2FGC, is responsible for transporting lipopolysaccharides, the main component of the outer membrane, from the inner membrane to the outer membrane in gram-negative bacteria. The complex forms a transport channel between the inner membrane and the outer membrane of the bacteria, extracts the lipopolysaccharide molecules from the outer leaflets of the inner membrane of the bacteria and initiates the configuration change of the protein peptide chains of nucleotide binding domains (NBDs) and transmembrane domains (TMDs) through ATP binding (non-hydrolysis), thereby squeezing lipopolysaccharides from the substrate binding cavity into the periplasmic domain (PDs), and finally transported by periplasmic proteins to the outer membrane to form a protective layer with drug resistance. The study revealed the mechanism of LptB2FGC recognition and transport of lipopolysaccharides, and showed that the two arginine rings at the interface of NBDs and TMDs play an important conformational communication function in transport. In this study, multiple functional sites involved in complex transport that can trigger bacterial death were identified through mutations. Therefore, these functional sites can be effective targets for novel antimicrobial designs. This research has important implications for the development of novel drugs to combat superbug infections.