
Recently, the team of Professor Haohao Dong from the State Key Laboratory of Biotherapy of West China Hospital published an article entitled "Structural insights into outer membrane asymmetry maintenance in Gram-negative bacteria by MlaFEDB" in the international authoritative journal Nature Structural & Molecular Biology Research papers. The research work was completed by the team of Professor Haohao Dong, the team of Professor Xing Zhang, director of the Cryo-EM Center of Zhejiang University School of Medicine, and the team of Professor Changjiang Dong of the University of East Anglia School of Medicine. Dr. Xiaodi Tang, associate professor of the State Key Laboratory of Biotherapy of our university, Dr. Shenghai Chang, engineer of the cryo-EM center of Zhejiang University, and Wen Qiao, a doctoral student of our university, are the joint first authors of the paper. The study was supported by the teams of Professors Xiawei Wei and Xiaofeng Zhu from Sichuan University. The State Key Laboratory of Biotherapy/National Clinical Research Center for Geriatric Diseases is the first author and corresponding author of the paper.
The study revealed four high-resolution cryo-EM structures in the bonded state of multidrug-resistant bacteria-gram-negative bacteria for transporting outer membrane phospholipid components to maintain the asymmetric structure of the outer membrane. Through structural analysis, in vivo functional studies, and in vitro phospholipid transport experiments based on fluorescence resonance energy transfer technology (FRET), the important functional sites of the protein machine and its high-efficiency transport mechanism were comprehensively elucidated.
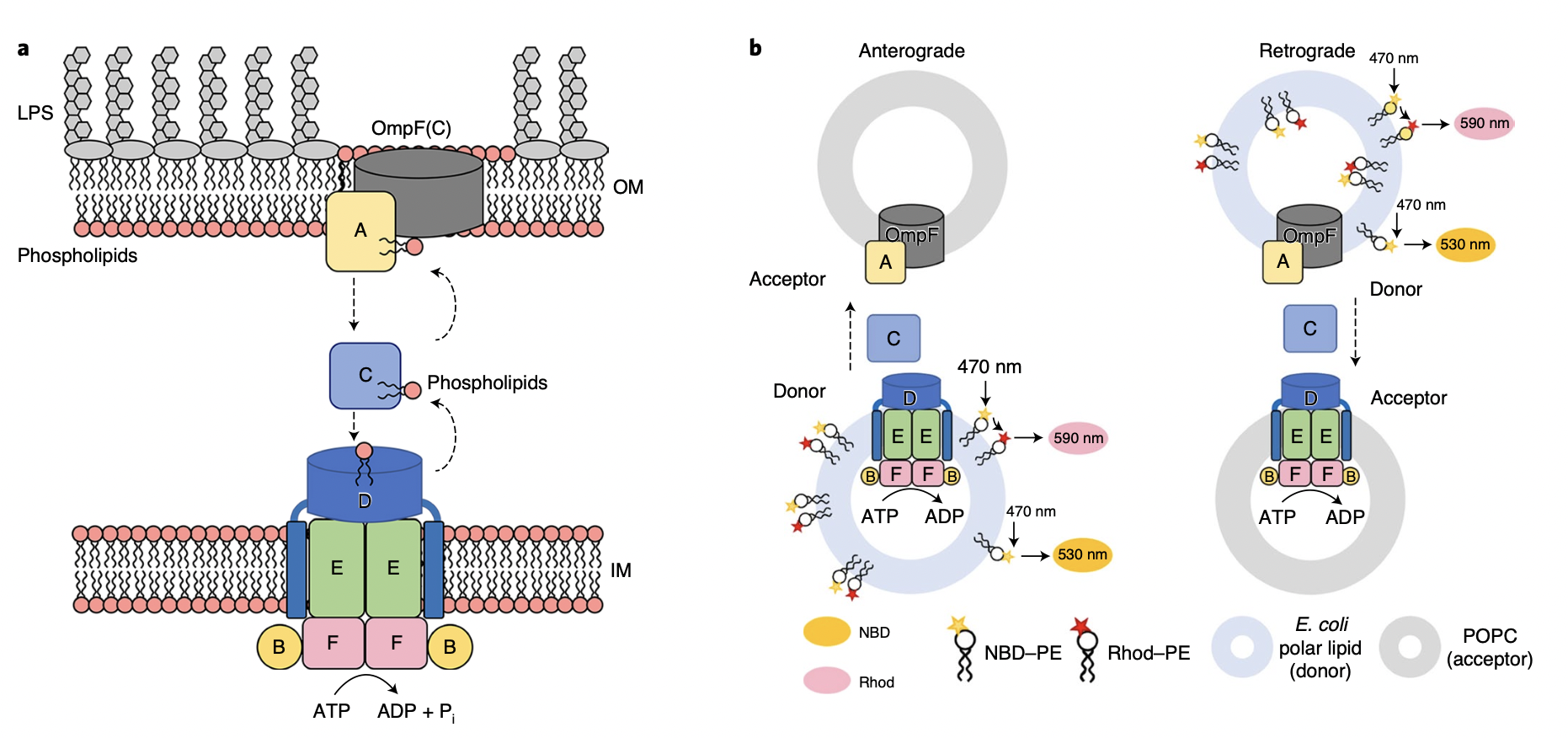
Drug resistance to bacterial infections is one of the major challenges in the medical community. Gram-negative bacteria (Pseudomonas aeruginosa, Enterobacteriaceae, Acinetobacter baumannii) are clinically common and the most concentrated pathogenic bacteria of drug-resistant bacteria. Their unique outer membrane structure effectively shields the entry of antibiotic drugs while allowing nutrients to enter, resulting in drug resistance. The selective permeability of the outer membrane of gram-negative bacteria is due to the asymmetric structure of the inner lobular phospholipid layer and the outer lobular lipopolysaccharide layer. Maintaining the asymmetry of the bacterial outer membrane is important for the survival of gram-negative bacteria. Asymmetric lipid maintenance protein MlaFEDBCA (Maintenance of lipid asymmetry) maintains the asymmetric structure of the outer membrane by recovering phospholipids erroneously distributed by bacteria in the outer outer membrane lobules, which plays an important role in maintaining the stability of the outer membrane of gram-negative bacteria.
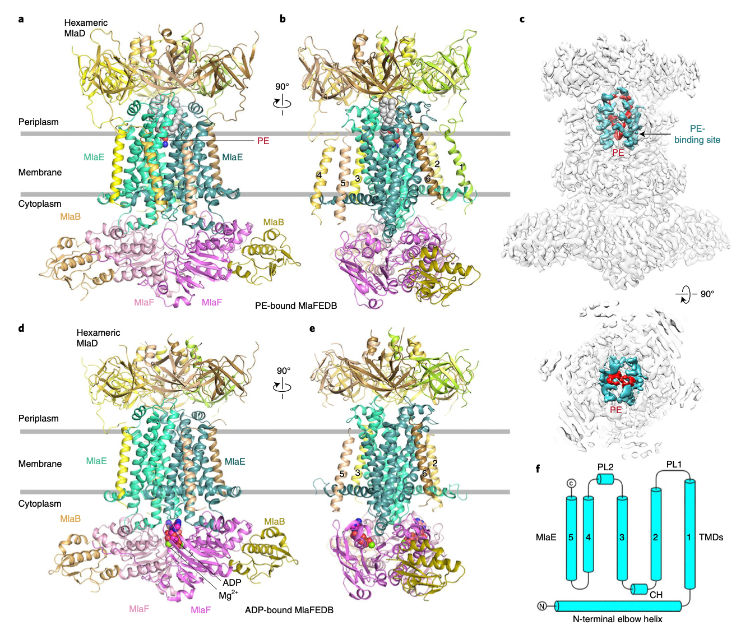
The complex forms a transport channel between the bacterial inner and outer membranes. First, the outer membrane protein complex MlaA/OmpF (C) transports phospholipids in the outer leaflets to the periplasmic protein MlaC, and MlaFEDB, as an ABC transporter protein, is located on the bacterial inner membrane, which is responsible for extracting the phospholipids bound by the periplasmic protein MlaC by ATP hydrolysis and energy consumption, and then transporting them to the inner membrane. Interestingly, the study found that the functional site of the binding phospholipid hydrophilic head in the substrate binding cavity of MlaFEDB is in the middle of the substrate binding cavity, and the amino acids on both sides are hydrophobic residues, which indicates that phospholipids will likely bind in both positive and negative ways. In vitro phospholipid transport experiments also confirmed the possibility of MlaFEDB participating in phospholipid bidirectional transport. The difference is that the inward transport of outer membrane phospholipids depends on ATP energy, while the outward transport of inner membrane phospholipids is a non-energy diffusion phenomenon. The protein machine has discovered bacterial ABC transporter proteins with potential bidirectional transport, although at this stage MlaFEDB is more believed to have its primary function of reverse transporting phospholipids in the outer membrane to the inner membrane by consuming energy to maintain the asymmetry of the outer membrane structure. In addition, mutation and transport experiments identified multiple functional sites that enhance bacterial drug sensitivity, which can be used as effective targets for novel antimicrobial designs. In short, this study opens up a new direction for the development of new drugs to fight superbug infection and has important guiding significance.