
On April 26, the team of Professor Haohao Dong and associate Professor Xiaodi'Tang from the State Key Laboratory of Biotherapy of West China Hospital of Sichuan University, together with the teams of Professor Xing Zhang and Professor Ruhong Zhou from Zhejiang University, jointly published a research paper entitled "Structural basis of BAM-mediated outer membrane β-barrel protein assembly" in Nature. Chongrong Shen, a graduate student of the "West China Biology National Innovation Class" of Sichuan University, Dr. Shenghai Chang of Zhejiang University, Qinghua Luo, a doctor/postdoctoral fellow of our university, and Jun Chen, a postdoctoral fellow of Zhejiang University, are the joint first authors of the paper. In addition, the research was strongly supported by the teams of Professor Xiawei Wei, Professor Xiaofeng Zhu, Professor Guangwen Lu of Sichuan University and Professor Changjiang Dong of Wuhan University. State Key Laboratory of Biotherapy, West China Hospital of Sichuan University/National Clinical Research Center for Geriatric Diseases, is the first author and corresponding author of the paper.
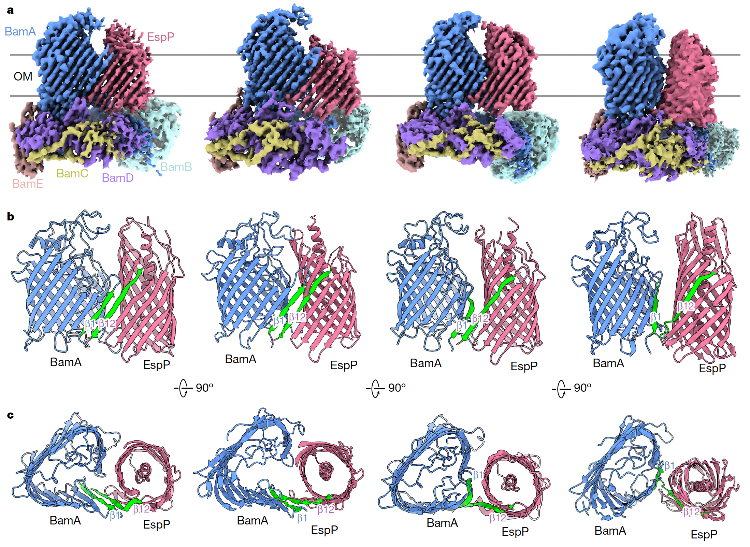
Figure 1. Cryo-EM density map and structural diagram of four dynamic intermediate states of BAM-EspP
This study captures for the first time multiple intermediate conformations of BAM and substrate β-barrel protein EspP in the process of outer membrane folding and integration (Figure 1), and reveals the whole process of substrate OMP assembly, closure and release through in vivo and in vitro functional analysis and all-atom molecular dynamics simulation, which provides an important scientific basis for the development of new antibacterial drugs targeting this protein complex.
Common clinical pathogens such as Pseudomonas aeruginosa, Klebsiella pneumoniae, Acinetobacter baumannii are gram-negative bacteria, and their special outer membrane components are the key to high pathogenicity and high drug resistance. β-barrel proteins (OMPs) are the main protein components of the bacterial outer membrane, which participate in the functions of bacterial membrane stability maintenance, host invasion and colonization, and drug resistance as a material exchange portal. The correct folding of the tertiary structure of the β-barrel protein is an important condition for its function: the new peptide chains are synthesized intracellular and transported to the outer membrane, where the β-barrel assembly machinery (BAM) helps fold into a barrel structure and integrate into the outer membrane. This process is very conserved in bacteria, and inhibition of BAM function can lead to bacterial death, so it is regarded as the most promising novel antimicrobial target. In recent years, the study of the structural function of BAM has become a hot topic in structural biology. It was found that the BAM protein was opened laterally by the core subunit BamA itself β-barrel without consuming energy, and the first β-sheet of BamA interacted with the last β-sheet (signal peptide) of the substrate OMP to initiate a dynamic molecular model of outer membrane protein folding. Previous studies have revealed the initial stages of BAM substrate integration, while the molecular mechanisms underlying how BAM folds, closes, and releases substrate β-barrels are unclear.
Since the process of assembling substrates in vivo is fast, capturing intermediate complexes of BAM integrated substrates is extremely challenging. The research team succeeded in obtaining the BAM-EspP complex protein by introducing cysteine at different locations in the interaction region predicted by BAM and EspP to spontaneously form disulfide bonds in vivo. The research team used single-particle cryo-EM technology to analyze the multiple conformations of BAM-EspP for the first time, including the high-resolution three-dimensional structure of the four states of open state, ready-to-close state, semi-closed state and full-closed state, showing the complete dynamic change process of BAM in the process of assembling substrate OMP. The working mechanism of BAM for EspP identification, integration and release is clarified (Figure 1).
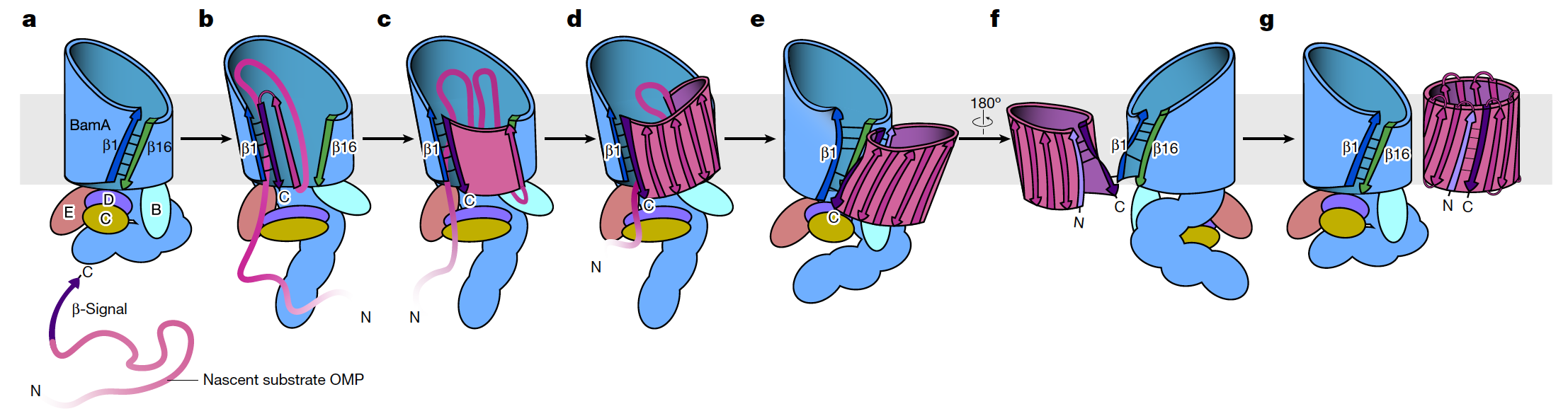
Figure 2. Schematic diagram of the working mechanism of the speculative BAM assembly substrate
BamA belongs to the OMP85 protein superfamily, which is involved in the assembly of proteins from bacterial outer membrane to human mitochondria and even plant chloroplast outer membrane proteins, and is evolutionarily conserved for β-barrel assembly function, indicating that these proteins share a common evolutionary origin and folding mechanism. The findings not only provide a scientific basis for the intervention of drug-resistant bacteria, but also have important implications for understanding the conservative folding and integration mechanism of β-barrel protein in eukaryotic double-membrane organelles, mitochondria and chloroplasts.
The research results were also invited to publish a research brief entitled "Step-by-step assembly of a β-barrel protein in a bacterial membrane" in the same journal Nature. The work of this project is all completed in China, and is funded by the key research and development of the Ministry of Science and Technology, the National Natural Science Foundation of China, the central financial funds, provincial and municipal projects, etc.
Haohao Dong, researcher and doctoral supervisor of the State Key Laboratory of Biotherapy and National Clinical Research Center for Geriatric Diseases, West China Hospital of Sichuan University, young chief scientist of the National Key R&D Program "Biomacromolecules and Microorganisms", and national overseas high-level young talents. The team has long been committed to target exploration and new drug research and development of drug-resistant bacteria, and has published 25 SCI papers in the research field of outer membrane formation and stability maintenance of gram-negative bacteria, including Nature (2014; 2016; 2023) 3 articles, Nature Communications (2017; 2019) and Nature Structural & Molecular Biology (2020; 2021) 2 articles. Related studies have analyzed the molecular mechanism of important functional protein machines and revealed important antibacterial targets, which is of great scientific significance for promoting the development of new antibacterial drugs.